|
||||||
|
Light
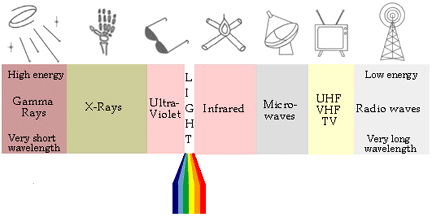
Ultraviolet <---- Visible Spectrum (350-780 nanoseconds) ----> InfraRed
Gamma Rays:
- The highest energy electromagnetic waves (or photons) are the
gamma rays.
- Application:Many nuclear reactions and interactions result
in the emission of
gamma rays. We can take advantage of these interactions for
medicinal purposes, especially in cancer treatments, where
focused gamma rays can be used to eliminate malignant cells.
- Application:Distant galaxies are also prodigious sources of
gamma rays,
where they are thought to be produced by very hot matter falling
onto a massive black hole in the center of the host galaxy. Our
atmosphere shields the Earth from most gamma rays produced in
deep space.
- Thewavelengths of these photons range from about 10^-8m to
10^-12m - The first discoverer of X-rays was Wilhelm Röentgen, a German
physicist who, in 1895, accidentally found these "light" rays when
he put a radioactive source in a drawer with some unexposed
photographic negatives and found the next day that the film had
been exposed somehow! The radioactive source had emitted
X-rays and produced bright spots on the film. - Application:X-rays are often used in medicine to see inside
the body, without
invasive surgery. The easiest method is to simply use
photographic paper behind the target (the body) and shine
X-rays through the target onto the film. Because the bones are
much denser than the rest of the tissue, the X-rays don't get
through them as well, so the bones show up as a dark areas on
the negative film. The rest of the tissue is relatively transparent to
the X-rays and show up as light areas on the film.
- The nearest high-energy neighbor to visible light is the
ultraviolet (UV) region - The sun is a strong emitter of UV radiation, but fortunately for
us, the Earth's atmosphere shields its inhabitants from the sun's
harmful UV light. In the upper atmosphere lies the ozone layer,
where most of the UV radiation is absorbed. - Application: Involved in photosynthesis for a number of plants
Visible:
- Occupying a very small part of the entire electromagnetic
spectrum, the visible light region is the only range of energies
that human eyes can detect.
- Just below (or just above, depending on which way you look at
it) the visible spectrum is infrared (IR) radiation. These are
longer wavelength light rays (about 1.00 mm to 8 x 10-7m, or
80,000Å) - Many molecules tend to resonate or vibrate easily when exposed
to IR radiation, so we erroneously think of IR as heat waves.
The sun emits about half of its radiation in the IR and even home
light bulbs emit more IR than visible light. - Applications:
- Many devices use IR as a means of transmitting information (IR
lasers, TV remote controls) and of receiving signals (IR
telescopes, photographic films, satellites). - In order to "see" at
night, IR goggles pick up the weak infrared radiation from our
warm bodies, translating the signal into visible light that our eyes
can detect. - Astronomers use IR telescopes to see inside and beyond huge
clouds of gas and dust that block our (visible light) view of such
places as the center of the Galaxy. - Art preservationists use IR cameras to view paintings that might
have underdrawings not
visible with the human eye
- Many devices use IR as a means of transmitting information (IR
Microwave:
- Microwaves is a misnomer. There's nothing "micro" about them,
in the scientific sense, which says that micro means "one
millionth". The wavelength range of this radiation is about 3.0 cm
to 1.0 mm - Application:Besides ovens, we use microwaves for many kinds
of
communication. Most television satellites and dishes operate in
the microwave region, and any kind of radar device is emitting
and receiving microwave signals. Short-wave radio operators
are using microwaves to talk to each other around the world,
because these waves can travel much further than weak radio
waves.
- UHF stands for ultra-high frequency and VHF
means very-high frequency. Considering the very low energies of
these waves (10-7eV to 10-5eV) which corresponds to a very
low frequency, it's hard to understand why someone called them
"ultra-high" and "very-high". UHF and VHF waves are
essentially just a-little-higher-energy radio waves. - Application: Your standard television antenna (the one
with the horizontal
metal rods, not the satellite dish) receives VHF and UHF waves
from the local television stations. The reason for the long rods?
Since these waves have wavelengths of a few centimeters to a
few meters, the electrons in the metal of the antenna need to be
able to vibrate with the same wavelength in order to "pick up"
the signal. Bunny ears on your old TV set usually aren't as long
as the rods on your roof-top antenna, so your reception
probably isn't as good. Plus, the metal in your house tends to
block and distort the signal, whereas non-conductors easily pass
the waves. Humans, on the other hand, act as a good conductor
for many TV waves, thus touching the antenna helps with the
reception.
Radio:
- The longest wavelength and smallest energy electromagnetic
wave (or photon) is the radio wave - Applications:
- AM signals are transmitted by radio and television stations
between 535 kHz and 1605 kHz, but can be picked up by
almost anything from standard telephones to orthodontia. - A possible frequence of a radio station might be to
transmits its signal is 91,500,000 Hz (Hz stands for Hertz, a unit
denoting cycles or waves/s). We know this as 91.5 MHz. This is
a frequency-modulated signal (FM) in which the frequency of the
waves are combined with the amplitude (the information) to
create the signal and eliminate noise, the major downfall of
amplitude-modulated (AM) signals.
- AM signals are transmitted by radio and television stations
Light Source: Characterized by the amount of energy it emits at every wavelength.
The spectrum of a light source shows is the distribution of light energy
vs. wavelength (lambda).
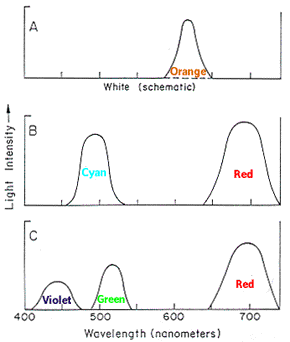
White Light = all visible wavelengths
 |
Here we can see the white light splitting into the spectrum using a prisim. |
Perception of Light:
- Brightness. = the amplitude of the spectrum.
- brightness adaptation:
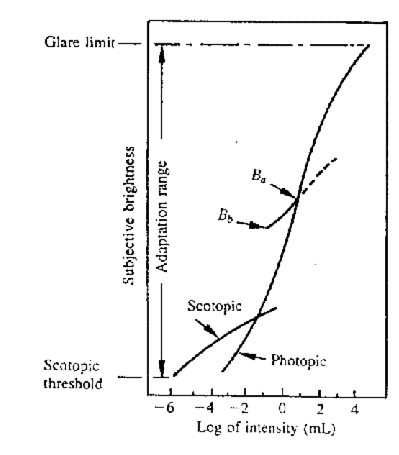
- brightness adaptation:
- Hue. Informally refered to as color.
- frequency componets of spectrum
- characterized by dominant frequency
- wavelength DOES NOT equal hue.
- Saturation. Spectral width, describes "whiteness" of a color
- unsaturated = a lot of white
- saturated = no white
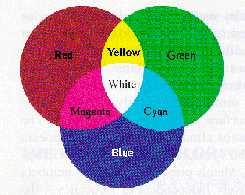
- Emitted Light
- The light from a monitor or projector. The primary hues are green, red, and blue. "Combining" any two opposite colors = white.
- Reflected Light
- Primary hues are yellow, cyan, and magenta. "Combining" opposites = black.